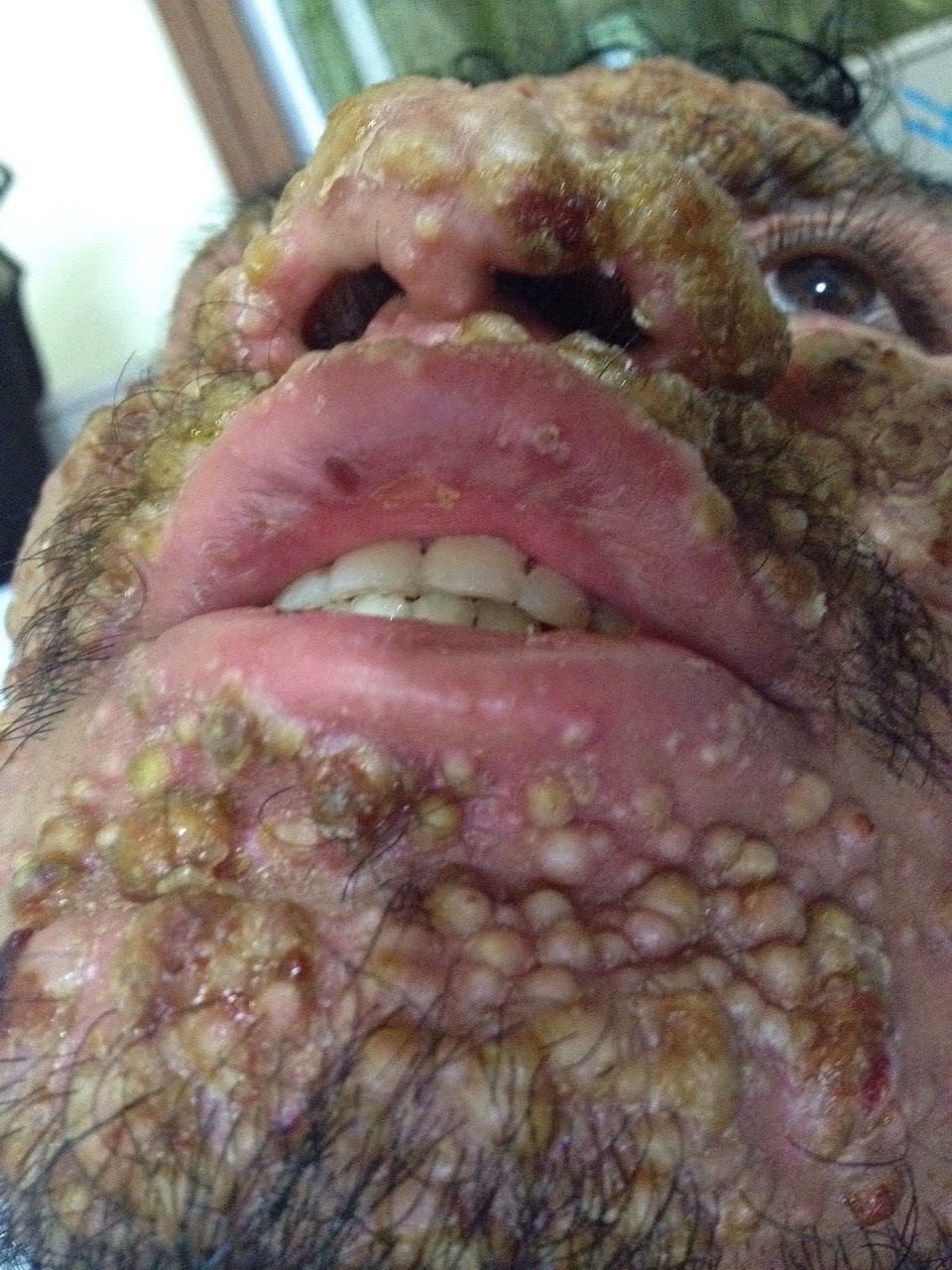
Histoplasma capsulatum is an airborne infection with a patchy global distribution. Particularly common among immunosuppressed patients, it presents with fever, weight loss, cough, skin lesions, abdominal discomfort or diarrhea, pancytopenia (low blood count) and abnormal liver tests.
In the Americas, more people with AIDS are killed by histoplasmosis than by TB. In hyperendemic regions (e.g. the Ohio and Mississippi River valleys) up to 60-90% of the population have been exposed to Histoplasma, with a mortality rate of ~4% for those with a symptomatic infection, according to the CDC.
However, it is difficult to diagnose via laboratory culture because the fungus is so slow-growing: historically, many patients die before the cultures have grown. Currently the most sensitive test is an enzyme immunoassay that detects Histoplasma antigen in the urine. In 85% of patients, a diagnosis can be made within the first 48 hours, allowing life-saving medication to be given in time. Commercial assays are available: an FDA-approved kit can be purchased from IMMY, or samples can be submitted to MiraVista Diagnostics.
Without antigen diagnosis, incorrect therapy with anti-TB therapy is the usual approach, with death in 1-3 weeks. As the average of death is 35 years and is avoidable, this represents an avoidable tragedy for all concerned.
Rolling out the antigen test across the world could save >48,000 lives over 5 years. On 9th July the WHO’s Essential Diagnostics List was updated to include the test, bringing its benefits a big step closer.

The application to include this test on the Essential Diagnostics List was made by Global Action Fund for Fungal Infections (GAFFI) following a meeting in Kampala (April 2018), and facilitated by publication of a meta-analysis of the value of Histoplasma antigen in AIDS, led by Professor Mathieu Nacher.
- Read the meta-analysis: Nacher et al (2018) PLoS Neglected Tropical Diseases
- Read more about the update to the Essential Diagnostics List
- Read a review on histoplasmosis diagnostics: Azar and Hage (2017) Laboratory Diagnostics for Histoplasmosis